To achieve healthy, vigorous plants for a pleasing landscape or a productive garden, the plants require optimum soil conditions that may be specific to the type of plant. Nutrient levels, drainage condition, and soil pH are examples of soil conditions that are important to consider when choosing plants and managing your landscape. This fact sheet discusses the pH factor alone; remember that many other factors are important for optimum growth.
Soil pH is a value that refers to the acidity or alkalinity level in the soil. Values of pH range from 0 to 14. A pH value of 7.0 is neutral, pH less than 7.0 is acidic, and pH greater than 7.0 is alkaline. The pH of soil is usually in the range 4.5 to 8.5. Most soils in New Jersey are naturally acidic. Some NJ soils are managed in such a way as to increase acidity—for optimum growth of acid-loving plant species, for example cranberry and blueberry crops. An extreme case of unintentional acidification may result from excavation & exposure of acid-producing sediments that lie in bands across NJ from NE to SW. On the other hand, some soils derived from limestone bedrock in the Ridge & Valley region of Northwest NJ may be near neutral or slightly alkaline. Furthermore, some NJ soils are alkaline because of excessive application of agricultural limestone or other liming amendment. The extreme cases of soil alkalinity in NJ usually result from excess (sodium) salt in the soil, either from de-icing salt, soluble fertilizer, or ocean water.
Soil pH is important to plants because of its effect on nutrient availability and the toxicity of related elements or ions. For example, the nutrient phosphorus is most available for plant uptake at pH 6.0 to 6.8. The element aluminum, which is not a nutrient but is a component of most soil minerals, becomes increasingly soluble and toxic at soil pH < 5. Certain nutrients also increase in availability for plant uptake as pH decreases, such as iron, manganese, and copper. These same nutrients could become unavailable (chemically "tied up") at pH 7.0 or above.
Most ornamental plants grown in NJ "prefer" soil pH in the range 6.1 to 6.8. In this range, the availability of nutrients is optimized for these plants, and aluminum toxicity is eliminated. However there are many ornamental plants, native or adapted to NJ, that exhibit optimum health/growth at lower pH (more acidic). This group is commonly referred to "acid-loving" and includes such favorite shrubs as azalea, rhododendron, holly, juniper, and mountain laurel. Some trees also fit into this category, for example pine, spruce, fir*, and some oak species. Heather, creeping phlox, and lily of the valley are a few of the perennials that are considered acid-loving.
It is important to know the target soil pH, the soil pH that is best suited for a plant. The following list specifies common landscape plants that are acid-loving. If you do not see a plant's name on this list, it is probably non-acid loving (optimal pH = 6.0 to 7.0).
*Douglas fir is not a true fir and does not fit into the "acid-loving" category.
Annuals adapted to acidic soil (pH 5.0 to 5.5)
- Ageratum, white
Herbaceous perennials adapted to acidic soil (pH 5.0 to 5.5)
- Alumroot, hairy
- Arnica
- Bleedingheart, fringed
- Bracken
- Bugbane, American
- Bunchberry
- Bunchflower
- Clubmoss, shining
- Crowberry
- Featherfleece
- Fern - Hartford, hay-scented, upland lady (but not Christmas, maidenhair, or walking fern)
- Fumitory, rock (but not climbing fumitory)
- Gayfeather
- Groundcedar
- Groundpine
- Heath
- Iris, Japanese
- Jack-in-the-pulpit, northern
- Ladyslipper, pink
- Lily-of-the-valley
- Meadowbeauty
- Milkweed, red
- Partridgeberry
- Phlox, creeping (not garden phlox)
- Pipsissewa
- Pitcherplant
- Polygala, fringed
- Pricklypear, common
- Pussytoes, common
- Rattlesnake-plantain - checkered, downy, or lesser
- Runningpine
- Sandmyrtle
- Spleenwort - Appalachian or lobed
- Springbeauty, Carolina
- Trailing arbutus
- Trillium, painted (not snow trillium)
- Turkeysbeard
- Turtlehead, pink
- Wild-indigo, yellow
- Wintergreen
- Woodfern, Clinton or mountain
- Woodsia, Rusty
- Woodsorrel
- Zenobia, dusty
Woody shrubs and ornamental trees adapted to acidic soil (pH 4.5 to 5.5)
- Andromeda (Pieris)
- Azalea
- Bayberry
- Blueberry
- Chokeberry
- Clethra
- Cranberry
- Daphne
- Fothergilla, dwarf
- Franklinia
- Fringetree, white
- Greenbrier - coral, laurel
- Hardhack
- Heather
- Hobblebush
- Huckleberry
- Holly, American
- Inkberry
- Jersey tea
- Juniper
- Kalmia, bog
- Labrador tea, true
- Lambkill
- Leatherleaf (viburnum)
- Leucothoe
- Laurel, mountain
- Magnolia
- Rhododendron
- Rhodora
- Scotchbroom
- Staggerbush
- Stewartia, mountain
- Sweetbay (magnolia)
- Sweetfern
- Withe-rod, smooth
- Yellow root
Large trees adapted to acidic soil (pH 4.5 to 5.5)
- Birch - sweet
- Cedar - white (but not red cedar)
- Chinquapin
- Cypress
- Hemlock - Carolina, common
- Fir (not including &qut;Douglas fir", which is not a fir at all)
- Mountain Ash, American
- Oak - post, scrub, southern red, or willow (but not black, chestnut, English, European turkey, pin, red, scarlet, swamp white, or white oak)
- Spruce
One of the main goals of soil testing is to measure the soil pH. Knowing the pH is necessary to determine whether soil amendments are necessary in a soil/planting area. If the soil is not in the optimum range recommended for a particular planting type, amendments can be made to improve the condition. The application rate (the amount needed per area) of the amendment depends on a complex combination of soil characteristics—mineralogy, soil texture, and organic matter content—which determine the soil's buffering capacity.
To adjust soil pH to a desired or target pH value, one must know not only the current soil pH but also the ability of the soil to resist pH change (soil buffering capacity). The soil buffering capacity is dependent on the cation exchange capacity of the soil, which quantifies the storage capacity of buffering ions. In the laboratory, an analytical technique is used to quantify the buffering capacity by introducing a chemical buffer solution (which strongly resists pH change) to a soil solution and determining the resulting pH. This is the principle of the Lime Requirement Index.
Rutgers Cooperative Extension Soil Testing Laboratory uses the Adams-Evans buffer solution (pH 8.0) to determine the LRI. Determinations of soil/buffer pH typically fall in the range 7.0 to 8.0, with the greater values representing poorly buffered soils (sandy, low organic matter) and the lesser values representing highly buffered soils (higher clay and/or organic matter content). When a soil pH is below the target pH for the desired planting, complex calculations that consider the initial soil pH, the target pH of the soil, and the buffering capacity of the soil are used to determine the amount of 'excess' acidity that needs to be neutralized, thereby creating recommendations for liming rate.
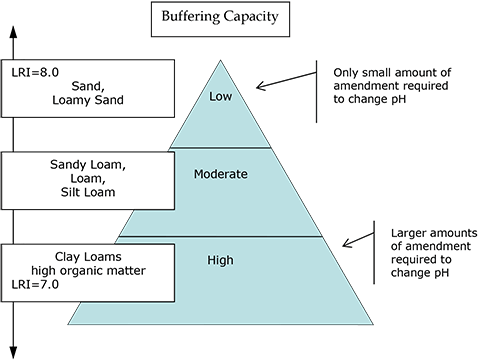
Figure 1. Generalized relationships of Lime Requirement Index, buffering capacity, soil texture, and rate of soil amendment required to change soil pH.
Your soil test report from Rutgers Soil Testing Lab states the pH of your soil sample, the Lime Requirement Index, and—if necessary—the recommended rate for limestone application to raise pH to an optimal level. In cases where magnesium is optimum or above optimum, calcitic limestone (maximum 40% calcium) is appropriate; on the other hand, magnesium deficiency (below optimum) would support use of dolomitic limestone (up to 13% Magnesium, 21% calcium). The soil test report will indicate if dolomitic limestone is warranted.
In some cases, the soil pH is higher than optimal for the desired planting. Then the soil test report may provide recommendations for lowering soil pH. In some cases where the pH is just slightly high, the recommendation will be for no limestone or other amendment, to allow natural processes to lower the soil pH with time. Certain nitrogen-containing fertilizers have an acidifying effect as well, and therefore the slightly above-optimum pH is likely to be corrected in relatively short term. The report will note that application of wood ashes, compost, etc. should be avoided; that is because those will tend to raise pH or suppress the change (drop) in pH. Over-application of limestone is the common cause of soil pH above 7.0; routine annual applications of limestone are discouraged unless repeated testing indicates the need (each soil and site will have different needs).
When soil pH is 7.5 or above, recommendations will indicate need for an acid-producing amendment. The preferred amendment for acidifying soil is powdered (elemental) sulfur. Sulfur is often found in the pesticide aisle of garden stores because of its fungicidal properties, but the label will also indicate its use for adjusting soil pH. Certain soil microorganisms oxidize the applied sulfur and produce acid in the process, which will lower the soil pH. Because it is a biological process, there is a lag time for the pH change that is dependent on weather & other environmental (growing) conditions. Under average conditions of moisture, temperature, and aeration (oxygen), count on at least six weeks' time for the change. A faster response will come from aluminum sulfate; in this case, the reaction is chemical and so the rate will depend on moisture and temperature to a degree, but not on biological metabolism & reproduction. Aluminum sulfate must be managed with care however, because soluble aluminum is toxic to plant roots and also has been implicated in human health problems. Gardeners should take care in using it (avoid dust, use gloves) and should apply it only to bare soil, planting at a later date (several days to one week should be adequate). Another amendment option for decreasing soil pH is sphagnum peat moss. No specific rates are available, but in general, a layer 2–3" thick mixed into 4–6" depth should improve conditions. Retest in one year to determine if additional amendment is required. (Do not use sphagnum peat moss as mulch; it becomes hydrophobic when dry and is likely to float away with any runoff).
In extreme cases of soil pH (above 8.0-8.3), soluble salts are implicated. Soluble salts include halite (NaCl), commonly used for de-icing sidewalks, driveways and parking lots, but also includes soluble fertilizers, for example from over-application, spreader malfunction, or a spill. Because of this, calcium chloride as a de-icing salt and slow-release fertilizers are recommended. The salts, because they are soluble, would eventually leach from the soil assuming adequate rainfall and drainage (and lack of further salt additions). However, there is no easy remedy for sodium salt-affected soils because the sodium disperses the physical structure of soil, impeding soil permeability, i.e., water flow through the soil. In such cases, replacement is one option; otherwise consult with your county extension agent.If pH is appropriate for the indicated planting but calcium is deficient, the soil test report will recommend agricultural gypsum (calcium sulfate) to boost the calcium level in the soil. Epsom salts (magnesium sulfate) and So-Po-Mag (potash of sulfate magnesia) are products that can build magnesium levels when limestone is not needed. Also keep in mind that N-P-K fertilizers may also contain some calcium and magnesium as components, for example calcium nitrate or calcium phosphate.

